Enhancing Vaccine Effectiveness With Lymph Node Expansion

By
Researchers have developed a biomaterial vaccine formulation that significantly enhances and extends lymph node expansion, leading to improved immune responses and vaccine efficacy against tumors. By jump-starting LN expansion before administering a traditional vaccine, they achieved more effective and sustained anti-tumor responses.
A new study reveals that enhanced lymph node expansion from biomaterial vaccines could boost tumor vaccine efficacy, potentially revolutionizing future vaccine developments.
The human body has approximately 600 lymph nodes (LNs). These small, bean-shaped organs house various types of blood cells and filter lymph fluid. Vaccines can cause the LNs near an injection site to temporarily expand, a phenomenon that is thought to reflect an ongoing vaccine immune response. Although researchers have studied the early expansion of LNs following vaccination, they have not investigated whether prolonged LN expansion could affect vaccine outcomes.
New Research Findings
Now scientists have found a way to enhance and extend LN expansion and study how this phenomenon affects both the immune system and efficacy of vaccinations against tumors. In a revolutionary approach, researchers used a biomaterial vaccine formulation that enabled greater and more persistent LN expansion than standard control vaccines.
While the oversized LNs maintained a normal tissue organization, they displayed altered mechanical features and hosted higher numbers of various immune cell types that commonly are involved in immune responses against pathogens and cancers. Notably, “jump-starting” lymph node expansion prior to administering a traditional vaccine against a melanoma-specific model antigen led to more effective and sustained anti-tumor responses in mice.
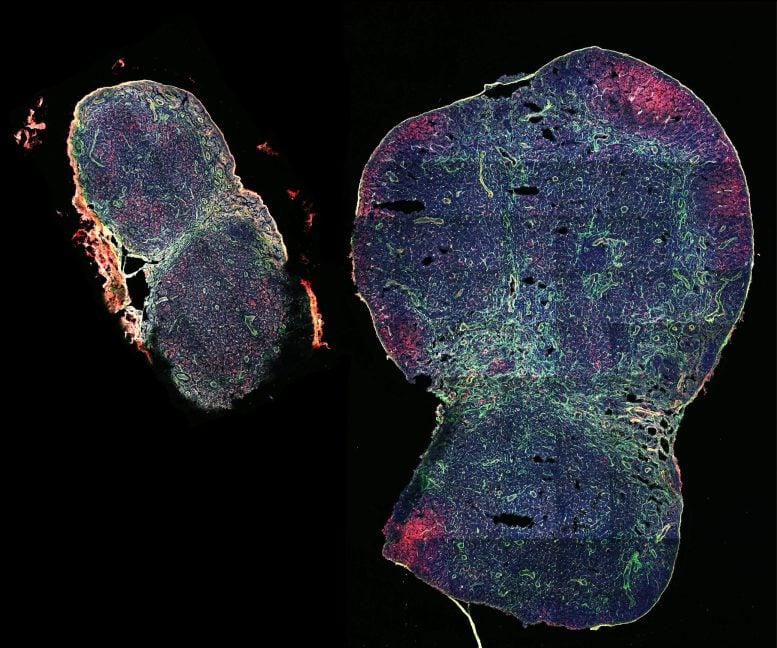
This immunofluorescent staining shows a lymph node that has been significantly expanded in mice with the help of the biomaterial MPS-vaccine (on the right), next to a lymph node taken from non-treated control mice (on the left) at the same time post-vaccination. Credit: Wyss Institute at Harvard University
This groundbreaking research was conducted by scientists from the Wyss Institute for Biologically Inspired Engineering at Harvard University, Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), and Genentech, a member of the Roche Group. The findings are published in Nature Biomedical Engineering.
Study Details and Implications
“By enhancing the initial and sustained expansion of LNs with biomaterial scaffolds, non-invasively monitoring them individually over long time periods, and probing deeply into their tissue architecture and immune cell populations, we tightly correlate a persistent LN expansion with more robust immune and vaccination responses,” said Wyss Institute Founding Core Faculty member David Mooney, Ph.D., who led the study. “This opens a new front of investigation for immunologists, and could have far-reaching implications for future vaccine developments.”
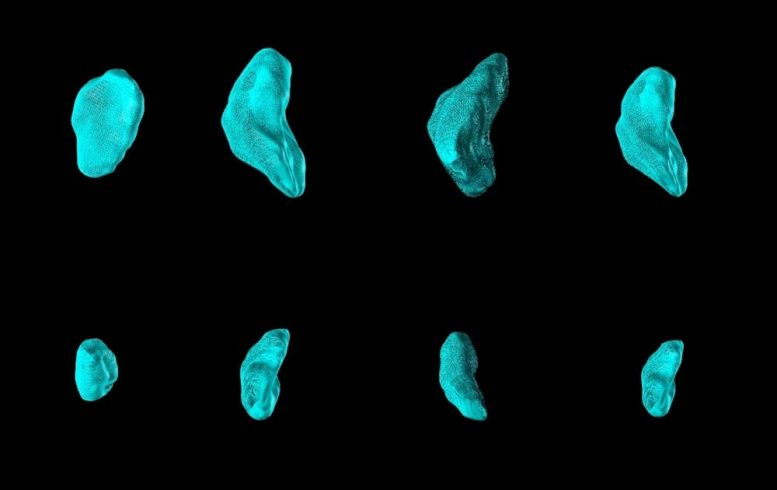
The authors used a method know as high-frequency ultrasound to monitor individual lymph nodes in MPS- and control-vaccinated mice. The top row shows a series of lymph nodes on day 7 following MPS vaccination. All of them were significantly expanded, compared to lymph nodes following control vaccination imaged at the same time and shown in the bottom row. Credit: Wyss Institute at Harvard University
Previous Work and New Discoveries
Mooney’s team at the Wyss Institute and SEAS had previously developed different biomaterial scaffolds as a matrix for cancer and infection vaccines. The researchers have demonstrated the potential of biomaterial vaccine formulations to successfully fight the growth of tumors in an extensive body of work performed in preclinical animal models and a first clinical trial with cancer patients. But they hadn’t yet investigated how their vaccines and those developed by others could influence the response of LNs draining leaked tissue fluid at vaccine injection sites, and have an impact on the LNs tissue organization, different cell types, and their gene expression, which could in turn affect vaccine efficacy.
In their new study, they tested a previously developed vaccine formulation that is based on microscale mesoporous silica (MPS) rods that can be injected close to tumors and form a cell-permeable 3D scaffold structure under the skin. Engineered to release an immune cell-attracting cytokine (GM-CSF), and immune cell-activating adjuvant (CpG), and tumor-antigen molecules, MPS-vaccines are able to reprogram recruited so-called antigen-presenting cells that, upon migrating into nearby LNs, orchestrate complex tumor cell-killing immune responses. Their new study showed that there are more facets to that concept.
“As it turns out, the immune-boosting functions of basic MPS-vaccines actively change the state of LNs by persistently enlarging their whole organ structure, as well as changing their tissue mechanics and immune cell populations and functions,” said first-author Alexander Najibi, Ph.D., who performed his Ph.D. thesis with Mooney.
Probing LNs With Ultra-Sound and Nano-Devices
To understand the response of LNs to MPS-vaccines over time, the team applied an ultra-sound imaging technique known as high-frequency ultrasound (HFUS). Similar to monitoring a tiny fetus developing in a mother’s womb by clinical ultra-sound, HFUS, on a much smaller scale, enables non-invasively and non-destructively monitoring of anatomical details of tissues and organs in small animals such as mice. Using HFUS, the team traced individual LNs in MPS-vaccinated mice over 100 days. They identified an initial peak expansion period that lasted until day 20, in which LN volumes increased about 7-fold, significantly greater than in animals that received traditional vaccine formulations. Importantly, the LNs of MPS-vaccinated mice, while decreasing in volumes after this peak expansion, remained significantly more expanded than LNs from traditionally vaccinated mice throughout the 100-day time course.
When Najibi and the team investigated the mechanical responses of the LNs using a nanoindentation device, they found that LNs in MPS-vaccinated animals, although maintaining an overall normal structure, were less stiff and more viscous in certain locations. This was accompanied by a re-organization of a protein that assembles and controls cells’ mechanically active cytoskeleton. Interestingly, Mooney’s group had shown in an earlier biomaterial study that changing mechanical features of immune cells’ environments, especially their viscoelasticity, affects immune cell development and functions. “It is very well-possible that in order to accommodate the significant growth induced by MPS-vaccines, LNs need to become softer and more viscous, and that this then further impacts immune cell recruitment, proliferation, and differentiation in a feed-forward process,” said Najibi.
From Immune Cell Engagement to Vaccine Responses
Interestingly, upon MPS-vaccination, the numbers of “innate immune cells,” including monocytes, neutrophils, macrophages, and other cell types that build up the first wave of immune defenses against pathogens and unwanted cells, peaked first in expanding LNs. Peaking with a delay were dendritic cells (DCs), which normally transfer information in the form of antigens from invading pathogens and cancer cells to “adaptive immune cells” that then launch subsequent waves of highly specific immune responses against the antigen-producing invaders. In fact, along with DCs, also T and B cell types of the adaptive immune system started to reach their highest numbers. “It was fascinating to see how the distinct changes in immune cell populations that we detected in expanding LNs in response to the MPS-vaccine over time re-enacted a typical immune response to infectious pathogens,” commented Najibi.
Innate immune cells and DCs are also known as “myeloid cells,” which are known to interact with LN tissue during early expansion. To further define the impact of myeloid cells on LN expansion, Mooney’s team collaborated with the group of Shannon Turley, Ph.D., the VP of Immunology and Regenerative Medicine at Genentech, and an expert in lymph node biology and tumor immunology. “The MPS-vaccine led to extraordinary structural and cellular changes within the lymph node that supported potent antigen-specific immunity,” said Turley.
By isolating myeloid cells from LNs and analyzing the gene expression profiles of individual cells (single-cell RNA-seq), the groups were able to reconstruct distinct changes in myeloid cell populations during LN expansion and identified distinct DC populations in durably expanded LNs whose changed gene expression was associated with LN expansion. In addition, the collaborators found that the number of monocytes increased 80-fold upon MPS-vaccination – the highest increase among all myeloid cell types – and pinpointed subpopulations of “inflammatory and antigen-presenting monocytes” as promising candidates for facilitating LN expansion. In fact, when they depleted specific subpopulations of these types of monocytes from circulating blood of mice after vaccination, the maintenance of LN expansion, and timing of the T cell response to vaccination, were altered.
Enhancing Vaccine Effectiveness
The team explored whether LN expansion could enhance the effectiveness of vaccination. “Jump-starting” the immune system in LNs with an antigen-free MPS-vaccine and subsequently administering the antigen in a traditional vaccine format significantly improved anti-tumor immunity and prolonged the survival of melanoma-bearing mice, compared to the traditional vaccine alone. “The priming of lymph nodes for subsequent vaccinations using various formulations could be a low-hanging fruit for future vaccine developments,” said Mooney.
“This newfound ability to physically expand lymph nodes and enhance their various immune activities over longer treatment courses, using cleverly designed and easy-to-administer biomaterials, could provide a tremendous push to immunotherapies in patients. It is also yet another great example of how mechanics plays a key role in regulation of living systems, even immune responses where few would consider physical cues to be important,” said Wyss Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and the Hansjörg Wyss Professor of Bioinspired Engineering at SEAS.
Reference: “Durable lymph-node expansion is associated with the efficacy of therapeutic vaccination” by Alexander J. Najibi, Ryan S. Lane, Miguel C. Sobral, Giovanni Bovone, Shawn Kang, Benjamin R. Freedman, Joel Gutierrez Estupinan, Alberto Elosegui-Artola, Christina M. Tringides, Maxence O. Dellacherie, Katherine Williams, Hamza Ijaz, Sören Müller, Shannon J. Turley and David J. Mooney, 6 May 2024, Nature Biomedical Engineering.
DOI: 10.1038/s41551-024-01209-3
Other authors of the study are Ryan Lane, Miguel Sobral, Giovanni Bovone, Shawn Kang, Benjamin Freedman, Joel Gutierrez Estupinan, Alberto Elosegui-Artola, Christina Tringides, Maxence Dellacherie, Katherine Williams, Hamza Ijaz, and Sören Müller. The study was funded by the National Institutes of Health/National Cancer Institute (award# U54 CA244726 and R01 CA223255).



