Scientists Unveil Groundbreaking Single-Molecule Detection Technique

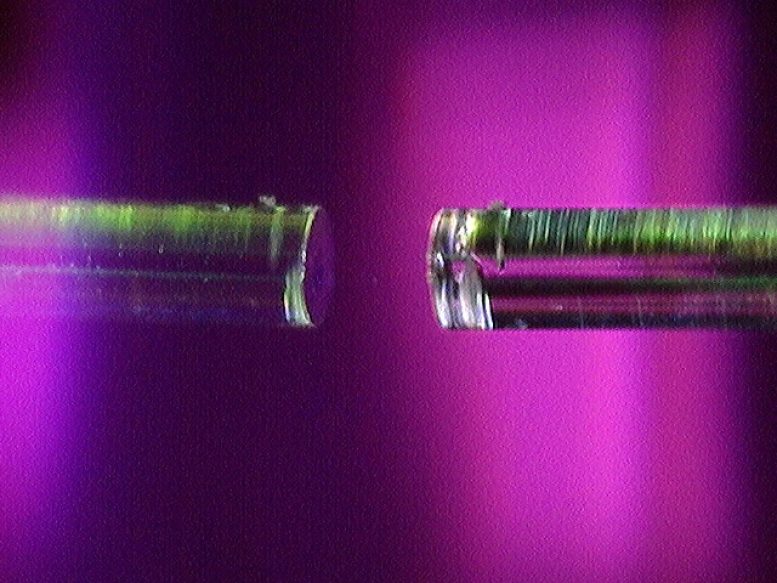
The heart of this study is a fiber microcavity. Here, one can see a small concave depression in the surface of an optical fiber. The researchers used a microcavity with two concave mirrors, but this image of a single concave microcavity makes it easier to see the fiber mirror setup. Credit: Photo by Carlos Saavedra / UW–Madison
University of Wisconsin–Madison scientists have developed a new, highly sensitive method to detect and analyze single molecules without using fluorescent labels, potentially transforming research in drug discovery and materials science.
Researchers at the University of Wisconsin–Madison have developed the most sensitive method yet for detecting and profiling a single molecule — unlocking a new tool that holds potential for better understanding how the building blocks of matter interact with each other. The new method could have implications for pursuits as varied as drug discovery and the development of advanced materials.
The technical achievement, detailed in a paper published in the journal Nature, marks a significant advance in the burgeoning field of observing individual molecules without the aid of fluorescent labels. While these labels are useful in many applications, they alter molecules in ways that can obscure how they naturally interact with one another. The new label-free method makes the molecules so easy to detect, it is almost as if they had labels.
“We’re very excited about this,” says Randall Goldsmith, a UW–Madison professor of chemistry who led the work. “Capturing behaviors at the level of single molecules is an amazingly informative way of understanding complex systems, and if you can build new tools that grant better access to that perspective, those tools can be really powerful.”
While researchers can glean useful information from studying materials and biological systems at larger scales, Goldsmith says that observing the behavior of and interactions between individual molecules is important for contextualizing that information, sometimes leading to new insights.
“When you see how nations interact with each other, it all comes down to interactions between individuals,” says Goldsmith. “You wouldn’t even think of understanding how groups of people interact with each other while ignoring how individuals interact with each other.”
The Importance of Single Molecule Observation
Goldsmith has been chasing the allure of single molecules since he was a postdoctoral researcher at Stanford University more than a decade ago. There, he worked under the chemist W.E. Moerner, who received the Nobel Prize in chemistry in 2014 for developing the first method of using light to observe a single molecule.
Since Moerner’s initial success, researchers around the world have devised and refined new ways to observe these tiny bits of matter.
The method that the UW–Madison team developed relies on a device called an optical microresonator, or microcavity. As its name suggests, the microcavity is an extremely tiny space where light can be trapped in both space and time — at least for a few nanoseconds — where it can interact with a molecule. Microcavities are more commonly found in physics or electrical engineering laboratories, not chemistry labs. Goldsmith’s history of combining concepts from disparate scientific fields was recognized in 2022 with a Polymath award from Schmidt Futures.
Microcavities are built from incredibly small mirrors fashioned right on top of a fiber optic cable. These fiber optic mirrors bounce the light back and forth many times very quickly within the microcavity.
Potential Applications and Future Developments
The researchers let molecules tumble into the cavity, let the light pass through it, and can not only detect the molecule’s presence, but also learn information about it, such as how fast it moves through water. This information can be used to determine the molecule’s shape, or conformation.
“Conformation at the molecular level is incredibly important, particularly for thinking about how biomolecules interact with each other,” says Goldsmith. “Let’s say you have a protein and you have some small-molecule drug. You want to see if the protein’s druggable, which is to say, ‘Does the drug have some kind of major interaction with the protein?’ One way you might be able to see that is if it introduces a conformational change.”
There are other ways to do that, but they require large amounts of sample material and time-consuming analyses. With the newly developed microcavity technique, Goldsmith says, “We can potentially build a black-box tool to give us the answer in tens of seconds.”
The team, which included Lisa-Maria Needham, a former postdoctoral researcher who is now a laboratory director at the University of Cambridge, has filed a patent for the device. Goldsmith says the device and methods will now be refined over the next couple of years. In the meantime, he says he and his collaborators are already thinking about the many ways it could be useful.
“We’re excited about many other applications in spectroscopy,” he says. “We hope we can use this as a stepping stone to other ways to learn about molecules.”
Reference: “Label-free detection and profiling of individual solution-phase molecules” by Lisa-Maria Needham, Carlos Saavedra, Julia K. Rasch, Daniel Sole-Barber, Beau S. Schweitzer, Alex J. Fairhall, Cecilia H. Vollbrecht, Sushu Wan, Yulia Podorova, Anders J. Bergsten, Brandon Mehlenbacher, Zhao Zhang, Lukas Tenbrake, Jovanna Saimi, Lucy C. Kneely, Jackson S. Kirkwood, Hannes Pfeifer, Edwin R. Chapman and Randall H. Goldsmith, 8 May 2024, Nature.
DOI: 10.1038/s41586-024-07370-8
This research was primarily funded by the National Institutes of Health (R01GM136981), with resonator construction supported by the Q-NEXT Quantum Center, a U.S. Department of Energy, Office of Science, National Quantum Information Science Research Center, under award number DE-FOA-0002253.


