Punjab orders immediate recall of unsafe medicines from pharmacies
LAHORE: The Punjab government has ordered the immediate withdrawal of several medicines from the market after they were found to be substandard and adulterated.
In an urgent alert issued in Lahore, the Directorate of Drugs Control Punjab directed all retailers, wholesalers, and distributors to suspend the sale and use of the identified products without delay. The department instructed all holders of the affected medicines to report their existing stock and consumption details to their respective area drug inspectors immediately.
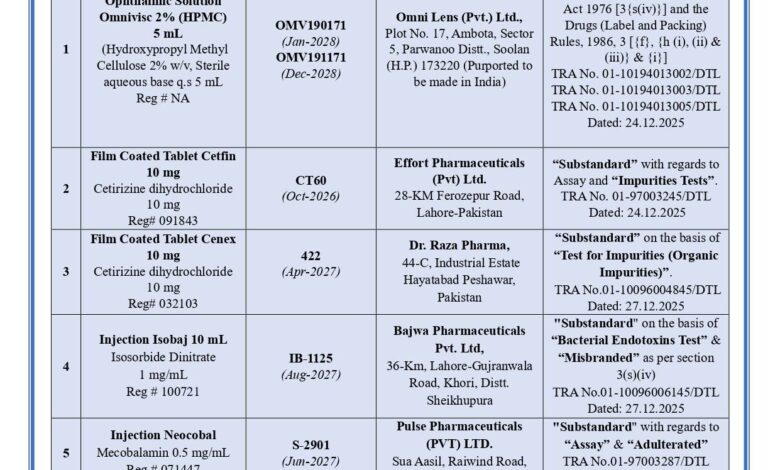
According to the official statement, the medicines were declared substandard and adulterated by the Drug Testing Laboratories Punjab. Following these findings, the Provincial Quality Control Board ordered their immediate recall from the market to protect public health.
Among the recalled products is Omnivisc 2% (HPMC) Ophthalmic Solution, 5 mL, specifically Batch No. OMV190171 with expiry in January 2028 and Batch No. OMV191171 with expiry in December 2028. These batches failed sterility tests and were also declared misbranded under the Drugs Act, 1976.
Other medicines declared substandard include Cetfin 10 mg film-coated tablets and Cenex 10 mg film-coated tablets. The recall order also covers several products classified as adulterated or substandard, including Isobaj Injection (10 mL), Neocobal Injection, Megadip 5 mg tablets, Kamedex Injection (1 mL), and Ascard-75 enteric-coated tablets.
The Directorate of Drugs Control Punjab has instructed all stakeholders to strictly comply with the recall directive, stressing that failure to do so could pose serious risks to public health and safety.

Source link



