Scientists Find a Promising Way to Fight a Deadly Superbug

Trinity College Dublin researchers have developed a promising approach to combat MRSA by targeting the immune suppressor IL-10 during vaccine delivery. As MRSA continues to evade traditional antibiotics, this innovative strategy offers new hope for effective vaccines against S. aureus infections, which are major contributors to mortality worldwide.
Researchers have made significant progress in the fight against MRSA by enhancing vaccine efficacy through targeting IL-10, an immune-suppressing molecule. Their findings suggest that neutralizing IL-10 can potentiate the immune response and aid in the clearance of the bacterium in animal models.
Scientists from Trinity College Dublin have taken a leap forward in understanding how we might fight back against the potentially deadly MRSA bacterium. They have shown in an animal model that targeting a key suppressive immune molecule (IL-10) during the delivery of a vaccine improves the ability of the vaccine to protect against infection.
The bacterium Staphylococcus aureus is one of the leading causes of community- and hospital-acquired bacterial infection, and is associated with over one million deaths worldwide each year. Unfortunately, antibiotics are becoming increasingly less effective against this bacterium with the antibiotic-resistant form, MRSA, responsible for the highest number of deaths in high-income countries that are attributable to antimicrobial-resistant bacterial infections.
Immunological Insights and Vaccine Enhancement
As a result, scientists are keenly focused on finding solutions to turn the tide in fighting S. aureus-related infections. One hugely appealing option is a vaccine but, while some progress has been made on that front in recent years, a number of major hurdles remain. One of these appears to be the bacterium’s ability to dampen the immune response by turning on one of the natural breaks that exists within the immune system, an important immune-suppressive molecule known as Interleukin-10 (IL-10), which acts to reduce inflammation in the body.
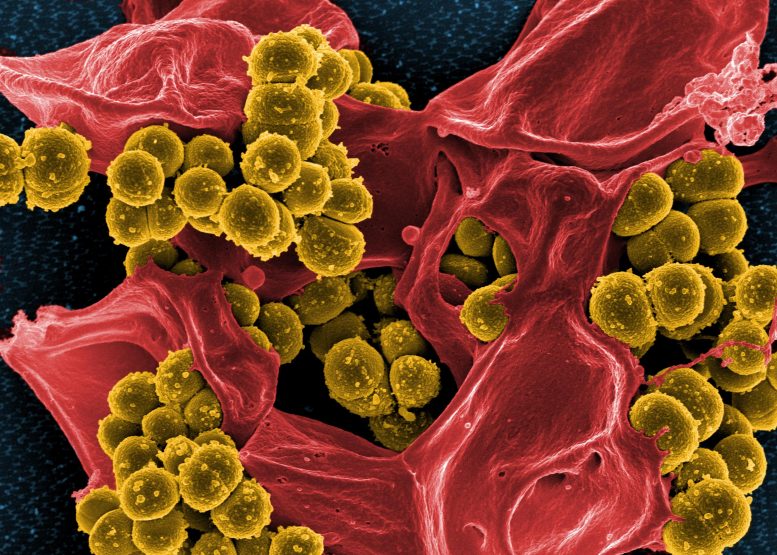
Digitally colorized scanning electron microscopic image of Staphylococcus aureus bacteria (mustard-colored) enmeshed within a human white blood cell (red-colored). Credit: NIAID
The interesting thing about S. aureus is that in addition to being a deadly pathogen, forms of this bacteria live in and on our bodies without causing harm. During these asymptomatic interactions the bacterium is, however, shaping the immune response – meaning that when a vaccine against S. aureus is administered the immune system struggles to respond appropriately.
Promising Results
Here, in the work published today (July 8) in a leading journal, JCI Insight, the researchers showed in the animal model that if they immunized subjects with a vaccine that primed their immune systems to respond to infection in tandem with antibodies that neutralized IL-10, the immune response (via specialized T cells) was improved and bacterial clearance was likewise improved following subsequent infection.
Staphylococcus aureus is a common bacterium that can be found on the skin and in the noses of many people. While typically harmless, it can cause a range of mild to severe infections if it enters the body through cuts or other wounds. Infections can include skin issues like boils and impetigo, and more serious problems such as pneumonia, bloodstream infections, and endocarditis. A particularly concerning aspect of Staphylococcus aureus is its ability to develop resistance to antibiotics, notably seen in MRSA (Methicillin-resistant Staphylococcus aureus), which is difficult to treat and is known for causing severe hospital-acquired infections.
Conclusion and Future Implications
The research team was led by Rachel McLoughlin, Professor in Immunology in Trinity College Dublin’s School of Biochemistry and Immunology. Rachel, who is based at the Trinity Biomedical Sciences Institute, said: “Taken in combination, our results offer significant promise for what would be a novel strategy for improving the efficacy of vaccines developed with the aim of suppressing S. aureus infection.
“Our work also strongly suggests that prior exposures to this bacterium may create a situation whereby our immune system no longer sees it as a threat and thus does not respond appropriately to a vaccine due to the creation of this immune-suppressed state. Again, this underlines why immunization delivered with something that helps neutralize IL-10 offers renewed hope for effective vaccines against S. aureus.”
Reference: 8 July 2024, JCI Insight.
Funding: Wellcome Trust, Irish Research Council, Science Foundation Ireland


