New Phosphorescent Probe Unmasks Microstructures in Water Ice

New research using phosphorescence spectroscopy shows how small organics like ethylene glycol disrupt the crystalline structure of water ice, offering insights into ice’s physical and chemical properties. Credit: Prof. Guoqing Zhang’s team
New research using phosphorescence spectroscopy shows how small organics like ethylene glycol disrupt the crystalline structure of water ice, offering insights into ice’s physical and chemical properties.
Ice is believed to have played a crucial role in the emergence of life. One reason is that organic molecules can be incorporated into the gaps between the crystal lattice by orderly arranged water molecules, leading to the concentration of organic compounds. However, current methods for studying organic molecules in ice, such as Raman and infrared spectroscopy, are mainly limited to absorption-based spectroscopic techniques, restricting measurement sensitivity.
A research team led by Prof. Guoqing Zhang, Prof. Shiyong Liu, Prof. Xiaoguo Zhou, and Researcher Xuepeng Zhang from the University of Science and Technology of China (USTC) developed a water-ice microstructures detection method using organic phosphorescent probes and phosphorescence spectroscopy. Their works were published in Angewandte Chemie.
A New Method for Analyzing Ice’s Organic Molecules
The team proposed an emission-based method to study organic molecules in water ice. They used the hydration state of a phosphorescent probe, acridinium iodide (ADI), to indicate the microstructural changes of water ice (i.e., crystalline vs. glassy). The microstructures of water ice can be significantly dictated by a trace amount of water-soluble organic molecules. Specifically, if water ice maintains amorphous at low temperatures, the AD+ cation and I– anion of the ADI probe will be separated by bound water molecules, exhibiting long-lived phosphorescence and a visible greenish-yellow afterglow. While in ordered crystalline ice, ADI probe molecules aggregate, inducing short-lived red phosphorescence through the heavy atom effect of iodine.

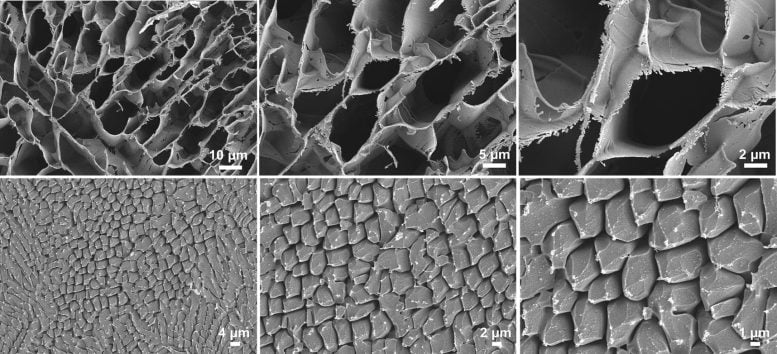
The Raman spectroscopy and cryoSEM Images of the ADI aqueous system. Credit: Prof. Guoqing Zhang’s team
Spectral Analysis Enhances Understanding of Ice Microstructures
The emission spectra revealed distinct spectroscopic changes in aqueous solution of ADI upon the addition of ethylene glycol (EG) small molecules and monodispersed EG polymers (PDI=1). The addition of trace amounts of EG (0.1%) leads to the emergence of the fluorescence band around 480 nm, accompanied by more intense phosphorescence band with well-resolved vibronic progressions at 555, 598, and 648 nm. The spectral results indicated that the addition of EG led to the transformation of ADI molecules in water ice from undissolved aggregates to dissolved ion states.
Verifying Phosphorescence Findings With Advanced Imaging Techniques
To corroborate the conclusions of phosphorescence spectroscopy, low-temperature scanning electron microscopy (Cryo-SEM) images showed that the addition of trace EG into the water ice containing ADI resulted in local areas with porous microstructures. Meanwhile, low-temperature Raman (LT-Raman) spectra confirmed that the addition of trace EG was sufficient to cause a shift in the O-H vibration of water ice from a low-frequency crystalline state to a high-frequency glassy state.
Implications for Water-Ice-Organics Interaction Studies
This study discovered that adding trace amounts of small or large molecular organics to water can significantly inhibit the crystalline order of water ice by using more convenient and sensitive phosphorescence spectroscopy. Moreover, the phosphorescence spectroscopy can also reveal morphological differences in water-ice microstructures when trace organics with different structures and same concentration are added into water, which is consistent with Raman spectroscopy and scanning electron microscopy, providing a new technical mean for studying water-ice-organics interactions at lower concentration and wider temperature range.
Reference: “Water-Ice Microstructures and Hydration States of Acridinium Iodide Studied by Phosphorescence Spectroscopy” by Hongping Liu, Hao Su, Ning Chen, Jie Cen, Jiajia Tan, Baicheng Zhang, Xiaoyu Chen, Aoyuan Cheng, Shengquan Fu, Xiaoguo Zhou, Shilin Liu, Xuepeng Zhang, Shiyong Liu, Yi Luo and Guoqing Zhang, 11 April 2024, Angewandte Chemie International Edition.
DOI: 10.1002/anie.202405314


